US FDA 21 CFR 820.30 Design Control Requirements
FDA 21 CFR Part 820.30 outlines the requirements for design controls within the Quality System Regulations (QSR) for medical devices. Design control consultants specializing in compliance with this regulation play a critical role in assisting medical device companies to establish and maintain effective design control processes. Design control consultants specializing in FDA 21 CFR Part 820.30 provide critical expertise in establishing and maintaining compliant design control processes for medical device companies, ensuring the safety, effectiveness, and quality of their products.
What is US FDA 21 CFR 820.30 Design Control?
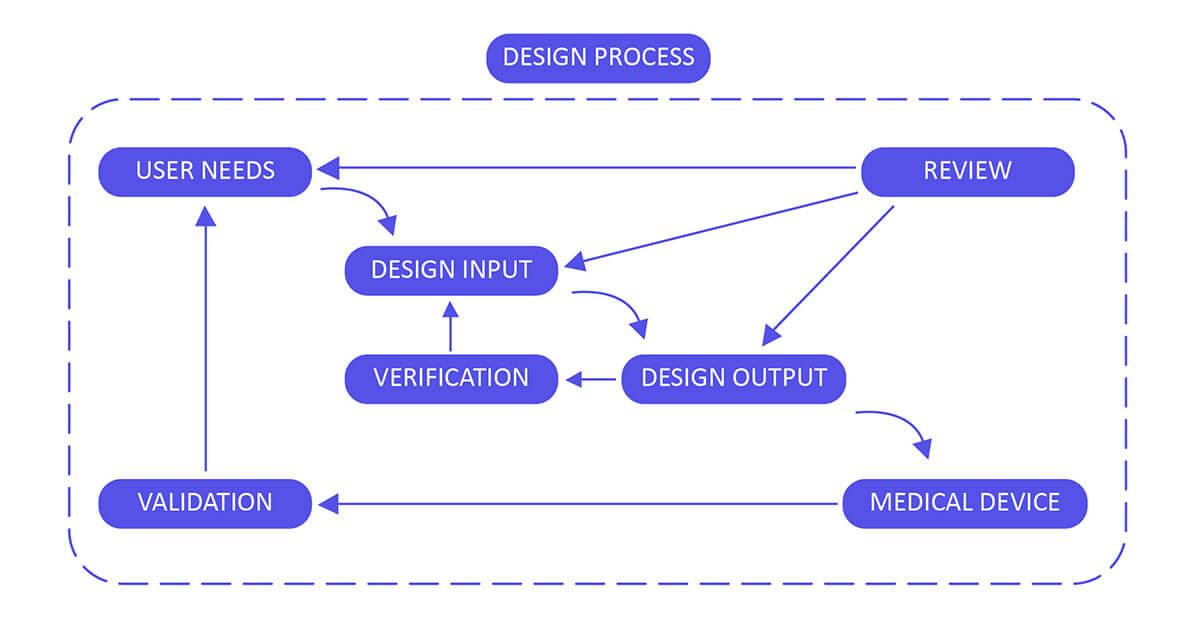
FDA 21 CFR Part 820.30 Design control requirements are the most important stage in the advancement of a medical device since a defective plan may prompt it to be inadequate or dangerous (that is, not affirmed or cleared by the administrative organization). At the design stage, an outline design control process should be started and actualized as a feature of the Quality System Requirement. Generally, outline design controls are straightforward and logical steps to ensure that what you develop is what you meant to develop and that the last item lives up to your client’s needs and desires.
We have experience with each constituent part and the GMP regulations that together form the basis for their development and manufacture: Drug Device Combination Products (FDA 21 CFR part 820) and (21 CFR Part 4).
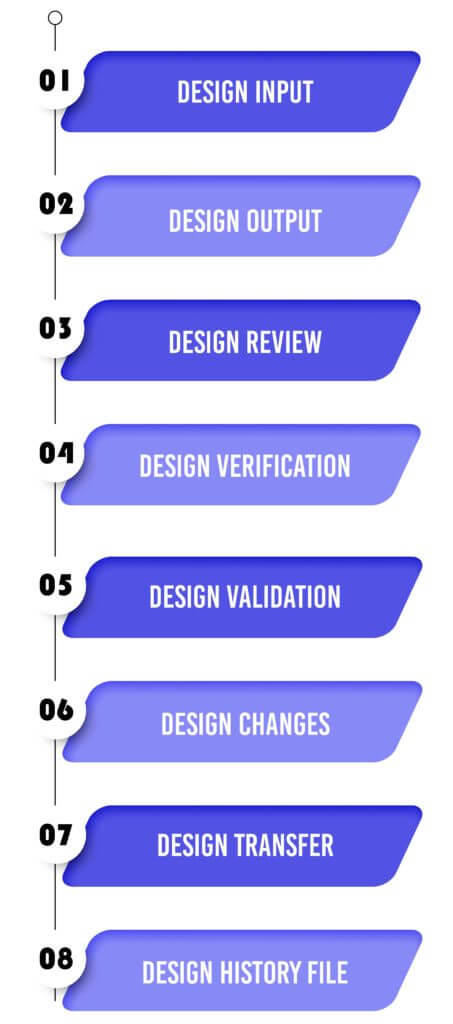
Design Control 21 CFR 820.30 Process for Medical Devices:
Build up and maintain a plan that describes the design and development activities and allocates the individual obligations for each activity. Guarantee you review, update and approve the plan until the device design is completed, verified and validated.
Design Input
Utilize performance, safety, business economics, outputs of risk management, and regulatory requirements as a basis to plan the device with the goal that its motivation and the proposed use are clear. The input may also come from surveying your customers(for example, clinicians, nurses, and patients).
Design Output
Design output methods or particulars need to stipulate or refer to the design input document developed by the team and need to identify the critical measures/outputs for the best possible capacity of the device. These incorporate the tests and strategies that may have been produced, adjusted or used to show conformance with the characterized configuration inputs. Examples of design outputs may include:
- The device itself.
- The user manual.
- Specifications A Risk Analysis Study results (For examples, validation and biocompatibility studies, storage).
- Technical Files.
Design Review
Confirm the design, or identify at an opportune time and right any insufficiencies distinguished at other plan and improvement phases. Two common types of review are hazard analysis, and failure mode and effect analysis.
Design Validation
Validate the device design through examination and targeted testing to ensure that the final design output consistently meets the specified intended use. Design validation should follow successful design verification. While design verification is conducted during the design process, design validation ensures that the medical device fulfills its intended purpose. Typically, this is achieved through in vitro performance, functional testing, animal testing, and/or in vivo clinical evaluations and trials.
Design Changes
Guarantee that all plan changes are distinguished, documented, approved, verified, reviewed and endorsed before usage.
Design Transfer
Ensure that the design of the medical device can be correctly translated into production specifications (that is, advancing successfully from product development to manufacturing).
Design History File
The design history file (DHF) aggregates confirm (that is, the history of the design) that demonstrates that the outline was created as per the outline of design control― specifically, the design and development plan, or the outline change design.
Operon Strategist is the leading medical device regulatory consultant providing consultation for 21 CFR 820.30 design control with extensive experience and the practical implementation of design controls regulation for developing new design control processes or for making improvements to existing processes.
We assist medical device manufacturers in the USA in design controls as per the US FDA and ISO 13485:2016 that can be mapped to the process that works best for the organization and the product being developed. If you need any help in setting up a design control system or wish to modify an existing system to align with ISO 13485 or FDA design controls, feel free to contact us.