Medical Device Design and Development Documentation Consultant in the USA
In the United States, consultants specializing in medical device design and development documentation provide critical support to companies aiming to create and maintain thorough documentation throughout the product development lifecycle. These consultants offer expertise in creating documentation that complies with regulatory requirements set by the FDA (Food and Drug Administration) and international standards.
Medical Device Design and Development
In order to meet particular healthcare demands, medical device design and development includes the development of new medical equipment or the improvement of preexisting ones. It involves more than only developing an idea, creating a working prototype, and then mass-producing it for sale. Delivering specialized, secure, and efficient solutions that satisfy user needs and legal requirements requires careful design and execution.

What is involved in the design and development of medical devices?
This procedure focuses on developing medical devices with specific goals, adhering to US FDA regulations, and determining their feasibility on the market. Important elements consist of:
Market Demand: Evaluating the demand for the device in the market to ensure its relevance and potential success.
Intended Impact: Understanding how the device is expected to impact healthcare and address specific medical requirements.
Safety of End Users: Prioritizing the safety of the device’s users, ensuring compliance with necessary safety standards and regulations.
This comprehensive process comprises multiple stages, including researching user needs, conceptualizing and designing the device, crafting prototypes, conducting rigorous testing, and securing regulatory approvals.
Looking for Design & Development Consultant?
Let’s have word about your project
What is the Process of Medical Device Design And Development?
During the design and development stage of the medical device design process, we assist various medical device manufacturing industries in USA to ensure that appropriate steps are taken to meet the regulatory compliance of the medical device design and development. After analyzing a new medical device, the next step in its product development is the medical device design. This is the most important stage in medical device development for a flawed design may ahead of it being ineffective or risky. At the medical device design stage, a design control process system is required.
Being, design controls are simple and logical steps to ensure that what you develop is what you determine to develop and that the final product meets your customer’s needs and expectations.
- Medical device development uses a ton of comparative parts in a large number of various medical devices. A solid definition extricated by dissecting the market needs. When you’re finished with the products definition and thought, you have to consider systems like FDA has characterized and licensed innovation rights. Medical devices classification depends on the hazard related to the utilization and upheld by law. So as to get into the market, the medical devices need to go through certain administrative compliances, subject to both provincial and worldwide guidelines. Medical devices measures are useful and upheld by law in indicating and assessing the prerequisite for structure and execution parameters for biomedical materials, apparatuses, and gear. These medical devices standards permit establishments in the medical device field, for example, products manufacturers, research centres, and others to review and survey such hardware and devices to guarantee standard quality and ease of use.
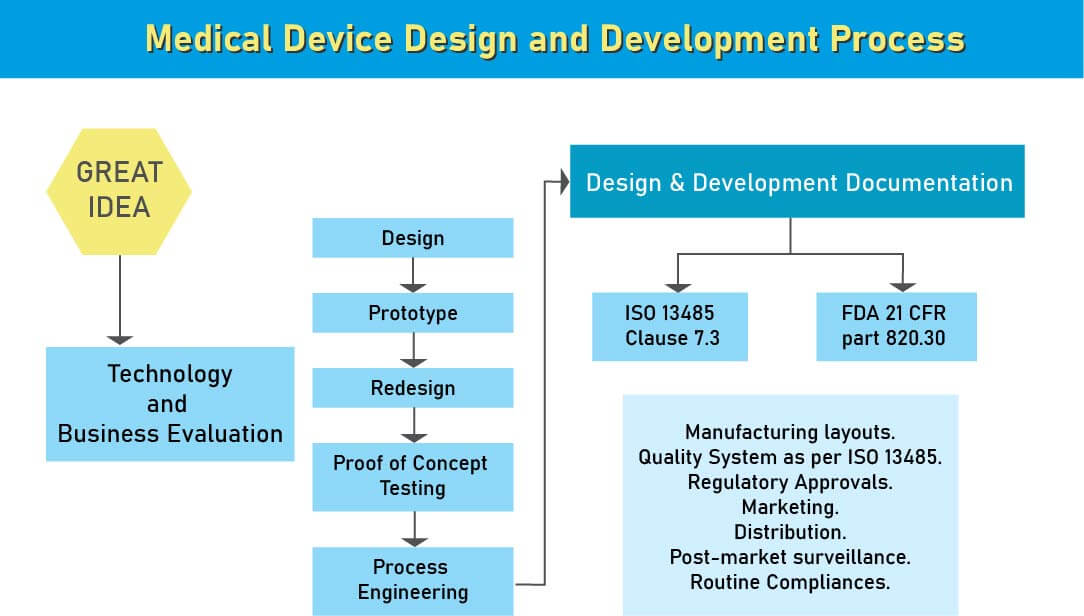
- The International Organization for Standardization likewise has details for medical device principles. ISO 13485 is broadly utilized guidelines over the world for medical device quality administration. Other than these worldwide models, there are sure gauges that are area explicit and every one of them is embraced by universal norms with little adjustment and constraint.
- Medical devices manufacturers need to pursue Design Control rules since the administrative bodies like FDA, European Commission, and others need to guarantee that the medical devices are alright for potential clients before makers begin to advertise the devices. Beginning stage from which Design Control starts is Design Input advancement and endorsement, which comprises of device design and manufacturing procedures to be completed in the generation stage. Design control is a comprehensive methodology and doesn’t end with moving the design to the generation stage when the plan is settled. It additionally affects manufacturing procedures as indicated by the adjustments in the design stage or even after creation input. It is a progressing procedure to build up a product that is usable for a client and in this way for the improved product, it considers progressive changes from utilization design just as breaking down failed items.
Why is Medical Device Design Important?
Medical device firms run the risk of suffering major setbacks or maybe failing to access the market if they do not have comprehensive design and development documentation. Thorough documentation guarantees:
- compliance with legal obligations.
- Good project management from conception to completion.
- streamlined procedures for regulatory approval.
The FDA’s required Design Controls offer a structured method for creating Class II and Class III medical devices that are registered under FDA 510(k) and need a lot of proof to prove their efficacy and safety.
What Do You Mean by Design Control Medical Device?
Design control, which are commanded by the FDA, address a formalized way to deal with the advancement of Class II and Class III medical devices under FDA 510k registration. This process incorporates many layers of required documentation that show the FDA precisely the way in which you have accommodated the wellbeing and viability of your new device. So it is important for medical device design and development companies to classify their product so that required design control can be done.
What is Product Development in Medical Devices?
Medical product development depicts the total course of fostering a medical device that beginnings with seeing a chance for a medical device to final selling the last tried and certified item. This process incorporates many stages, including opportunity identification, market examination, idea generation and determination, product plan, device designing, make commercialization and post-market surveillance.
How to Design and Develop a Medical Device?
It takes a significant amount of effort to deliver the right healthcare solution that meets customer demands. A right healthcare solution demands everybody staying on the same page, with strong scope definition from end user’s need, collaborative efforts across the team, adherence to specification and requirements extracted from product definition, simultaneously mitigating risks and sticking to the best possible quality.
Medical Device Product Development Process?
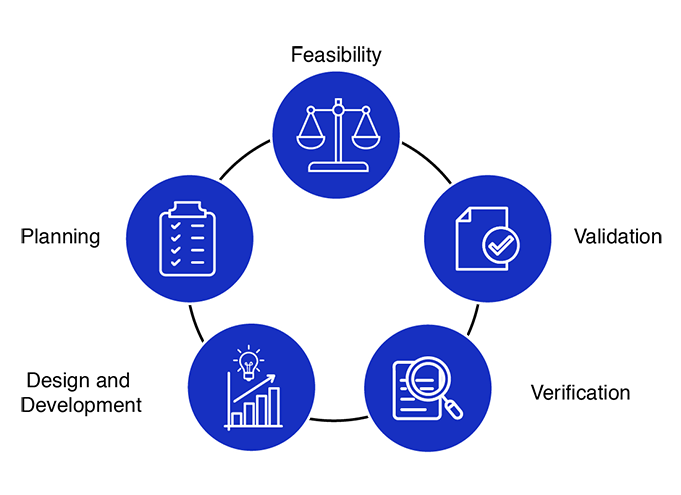
Medical device design services and development process encourages an early focus on clear problem definition and de-risking a wide variety of potential solutions. By later phases, the funnel of medical device design options narrows significantly, converging on a final product that has been thoroughly shown to meet the customer needs and is ready for distribution.
The Medical Device Design and Development Services Includes
Combination Product - Drug - Device
Each manufacturer of Drug Device combination product (e.g. Drug, device combination products like prefilled syringes, applicators of the tropical products) shall have adequate design and development activity done so as to prove the adequacy of the safety and efficacy of the product. The medical device design and development activity is the systematic methodology, which establishes the proper medical device design and development.
Medical Device Design Control
After conceptualizing a new medical device, the next step in its product advancement is the design. This is the most important stage in the advancement of a medical device since a defective plan may prompt it being inadequate or dangerous (that is, not affirmed or cleared by the administrative organization). At the medical device design stage, an outline control process should be started and actualized as a feature of the quality system requirement.
Operon Strategist’s Role in Device Design Control
At Operon Strategist, we assist medical device manufacturers by: Offering our proficiency in creating thorough and legally compliant design documentation.
- assisting producers with the FDA and global regulatory procedures.
- supporting risk management, QMS deployment, and design validation.
- delivering timely, error-free, and reasonably priced services that adhere to industry standards.
Successful product development and market entrance are made possible by working with knowledgeable consultants that guarantee smooth navigation through the intricate design and regulatory environment.
To help you meet your regulatory objectives and uphold quality perfection, get in touch with Operon Strategist for dependable and effective medical device design and development assistance.
FAQs
What Do You Mean by Design Control Medical Device?
Design control, which are commanded by the FDA, address a formalized way to deal with the advancement of Class II and Class III medical devices. This process incorporates many layers of required documentation that show the FDA precisely the way in which you have accommodated the wellbeing and viability of your new device. So it is important for medical device design and development companies to classify their product so that required design control can be done.
What is Product Development in Medical Devices?
Medical product development depicts the total course of fostering a medical device that beginnings with seeing a chance for a medical device to final selling the last tried and certified item. This process incorporates many stages, including opportunity identification, market examination, idea generation and determination, product plan, device designing, make commercialization and post-market surveillance.