ISO 13485 Certification in USA
ISO 13485 Certification Consultants in USA
Looking to achieve ISO 13485 certification in the USA? Operon Strategist helps medical device manufacturers, startups, and exporters establish and implement a compliant Quality Management System (QMS) aligned with ISO 13485:2016 standards.
As a trusted ISO 13485 Consultant in the USA, we ensure a faster, audit-ready, and cost-effective certification journey.
What is ISO 13485?
ISO 13485 is an internationally recognized standard for Quality Management Systems (QMS) specific to medical devices. It ensures your organization consistently meets regulatory, customer, and product quality requirements throughout the product lifecycle — from design and development to production and post-market surveillance.
Benefits of ISO 13485 Certification for USA-Based Companies
- Increased process control and product quality
- Easier access to international markets (including CE Marking and FDA clearance)
- Reduced product recalls and compliance risks
- Strengthened brand credibility and customer trust
- Better preparedness for FDA inspections and audits
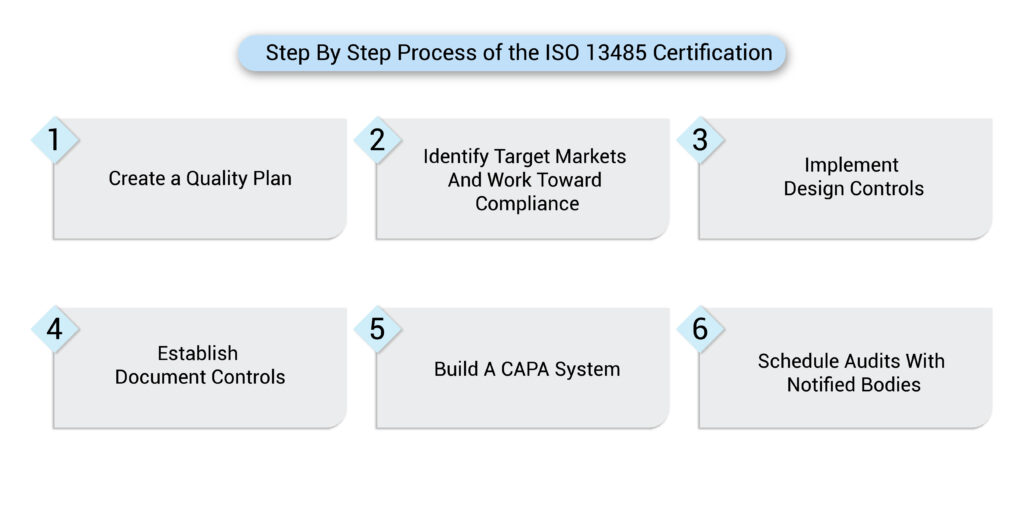
Our ISO 13485 Certification Process
Our ISO 13485 consulting service includes a step-by-step roadmap, tailored for medical device firms operating or expanding in the USA:
Step 1: Gap Analysis & QMS Planning
We evaluate your current processes and create a custom quality plan to bridge compliance gaps.
ISO 13485 Certification Consultants in USA
Step 2: QMS Development & Documentation
We assist in design control, risk management, CAPA, document control, and training programs aligned with ISO 13485.
Step 3: Internal Audit & Corrective Actions
Our team conducts internal audits to ensure you’re fully prepared before facing a Notified Body or third-party auditor.
Step 4: Certification Support
We coordinate with certification bodies, manage audit responses, and support post-audit improvements.
Key Areas of Our ISO 13485 Consulting Services
- Quality Manual & SOPs Development
- CAPA & Risk Management Integration
- Document Control System Implementation
- Regulatory Strategy (for FDA and international markets)
- Internal Audit Training & Execution
- Audit Preparation & Support
- Support for ISO 13485:2016 Transition
Get Expert Consulting Services of QMS for Medical Devices
Why Choose Operon Strategist as Your ISO 13485 Consultant in USA?
- 15+ years of regulatory and QMS experience in the medical device industry
- Specialized in FDA 21 CFR Part 820 and ISO 13485 integration
- Expertise in USA-specific regulatory expectations and global standards
- Custom QMS implementation and documentation support
FAQs
What is the purpose of ISO 13485 certification for a US-based company?
The purpose of ISO 13485 certification for a US-based company is to improve their quality management systems, enhance compliance with regulatory requirements, and potentially expand market access by demonstrating adherence to internationally accepted quality standards.
How can ISO 13485 certification help in international markets?
ISO 13485 certification is globally recognized and accepted as a benchmark for quality management in the medical device industry. Certification can facilitate market access in other countries and regions where ISO 13485 is widely recognized, streamlining the regulatory process and enhancing the credibility of the company and its products in international markets.