Introduction
Orthopedic implants play a crucial role in restoring mobility and improving quality of life for millions of patients across the U.S. Whether it’s a hip replacement, spinal fixation device, or trauma plate, these medical devices must be manufactured with precision, compliance, and innovation at the core.
What Are Orthopedic Implants?
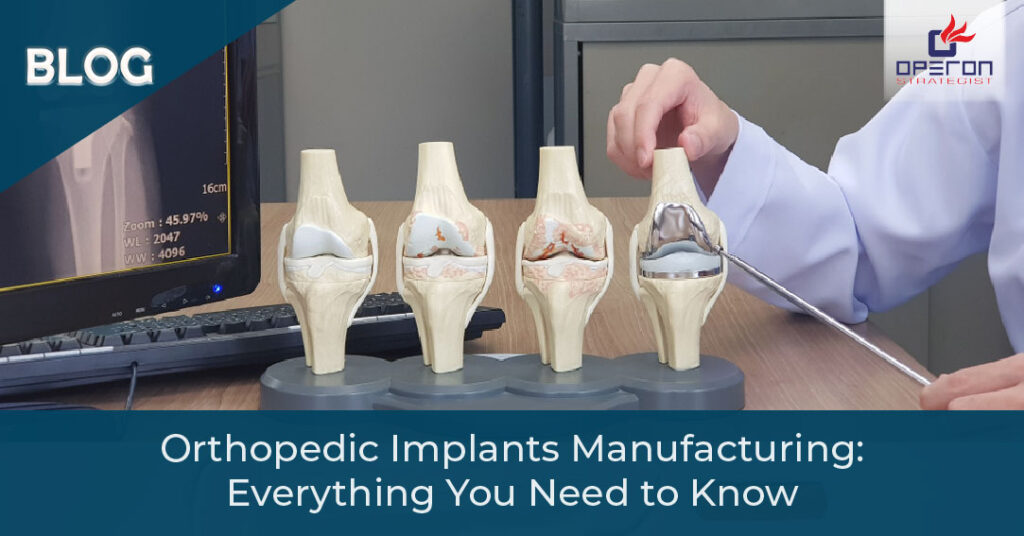
Orthopedic implants are medical devices used to replace or support bones and joints that are damaged due to injury, aging, or medical conditions like arthritis. Common types include:
- Joint replacement implants (hip, knee, shoulder)
- Trauma fixation devices (plates, screws, nails)
- Spinal implants (rods, cages, and screws).
- OrthoBiologics (materials that promote healing)
Each implant type has a specific application, and its manufacturing process must meet stringent U.S. FDA and ISO standards.
What materials are used in Orthopedic Implants Manufacturing?
Choosing the right material is critical for performance and patient safety. Common materials include:
- Titanium and its alloys – lightweight, corrosion-resistant, and biocompatible
- Stainless steel – strong and cost-effective
- Cobalt-chrome alloys – excellent wear resistance
- PEEK (Polyether ether ketone) – used for spinal cages
- Ceramics and composites – often used for joint surfaces
Manufacturers must ensure the material is tested, traceable, and suitable for long-term implantation.
Key Steps in the Orthopedic Implants Manufacturing Process
- Design & Prototyping
Using CAD software and 3D printing, manufacturers can create customized implants that meet both anatomical and biomechanical requirements. - Material Sourcing & Preparation
Raw materials are selected and shaped through forging, casting, or machining. - Precision Machining
CNC machines are used to ensure tight tolerances and surface finishes that meet implant specifications. - Surface Treatments
Coatings such as hydroxyapatite or plasma spray improve biocompatibility and encourage bone integration. - Sterilization & Packaging
The final product must be sterile and packaged in a way that maintains cleanliness and integrity during storage and shipping.
Regulatory Requirements for the U.S. Market
If you are manufacturing orthopedic implants for the U.S. market, FDA compliance is non-negotiable. Here’s what you need to know:
- 21 CFR Part 820 – Quality System Regulation (QSR):
Mandates a quality management system that covers design controls, process validation, CAPA, and more. - 510(k) Submission or PMA:
Most Class II implants require a 510(k) clearance. Novel implants may require a more rigorous Premarket Approval (PMA). - UDI Compliance:
Implants must be labeled with a Unique Device Identifier (UDI) to improve traceability. - ISO 13485 Certification:
While not mandatory in the U.S., it’s widely adopted by manufacturers to demonstrate QMS compliance.
Market Growth for Orthopedic Implant Products
The global orthopedic implants market is booming. According to industry reports, the market is projected to grow at a CAGR of over 4.8% between 2024 and 2030.
Key drivers of this growth include:
- Aging populations worldwide
- Rising incidence of orthopedic conditions like osteoporosis and arthritis
- Technological innovations (like 3D-printed implants)
- Expanding healthcare access in emerging markets
In the US, the demand for orthopedic implants remains strong, with increasing preference for minimally invasive procedures and personalized implant designs.
How Operon Strategist Can Help
At Operon Strategist, we support orthopedic implant manufacturers with:
- Manufacturing plant setup and documentation with machinery guidance
- ISO 13485 QMS implementation
- Design and development documentation
- Risk management and validation planning
- FDA 510(k) or CE marking submission and PMA consulting
We act as your end-to-end regulatory partner—guiding you from concept to commercialization.
Talk to Operon Strategist Now.
Why Getting It Right Matters?
Orthopedic implants manufacturing is a high-stakes, high-reward industry that demands precision, quality, and regulatory excellence. Whether you’re a startup or an established manufacturer, getting expert guidance can make all the difference in product success.
Contact Operon Strategist today to get started.

