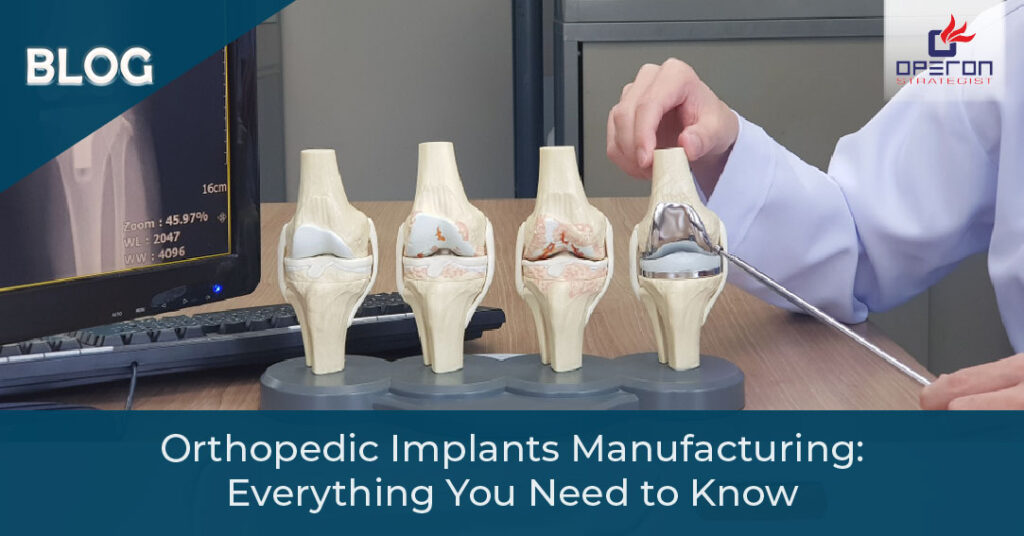
Intraocular Lenses Manufacturing
Introduction Intraocular lenses (IOLs) have revolutionized vision correction, especially for patients undergoing cataract surgery. As the demand for advanced ophthalmic devices rises in the USA, manufacturing intraocular lenses presents a significant opportunity—but it also comes with strict technical, regulatory, and quality requirements. This guide explores the complete IOL manufacturing process, critical FDA compliance standards, and […]
Intraocular Lenses Manufacturing Read More »