What is a US FDA 510(k) submission?
The US FDA 510(k)-submission process of the FDA serves as a barrier to ensure the quality and compliance of medical devices before they are allowed into the US market and used by American patients. The main objective of this process is to demonstrate “substantial equivalence,” meaning that the device being submitted is like a previously approved device known as a predicate device.
The rationale behind the US FDA 510(k) submission is to show that the device in question is comparable to the predicate device and therefore since the predicate device is already proven to be safe and effective, the submitted device must also be safe and effective.
How to Submit a US FDA 510(k)?
The process of submitting a US FDA 510(k) requires careful attention and time to ensure accuracy. There is no single form to complete and send to the FDA. Instead, specific documentation must be collected and submitted. The FDA has provided helpful guidance on how to prepare a formulated and tabulated 510(k), including information on the necessary content and format.
Submitting a US FDA 510(k) application is a multi-step process involving careful preparation and adherence to FDA regulations. Here’s an overview:
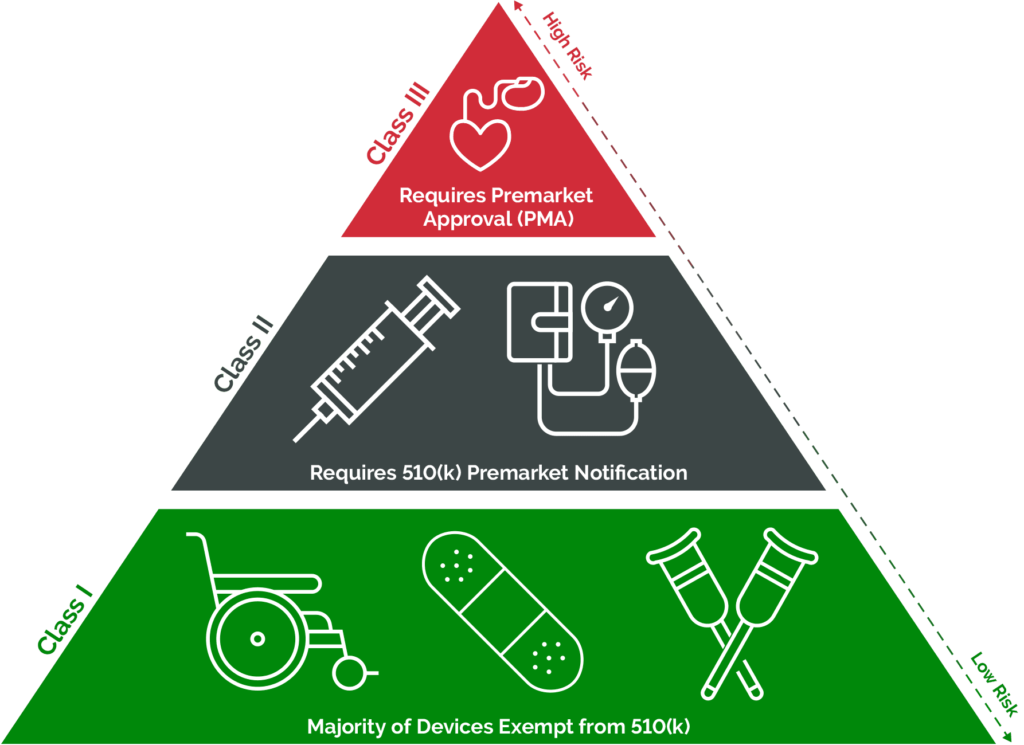
- Determine Device Classification: Classify the medical device according to the FDA’s classification system (Class I, II, or III). Manufacturers must understand the device classification and associated regulatory requirements.
- Identify Predicate Device: Select a legally marketed predicate device that closely matches your new device. This step requires choosing an appropriate reference device for comparison.
- Prepare Detailed Documentation: Compile comprehensive documentation including device design, performance data, labeling, instructions, risk analysis, drawings, and schematics. Proper documentation of device design and intended use is critical.
- Conduct Relevant Testing: Perform necessary testing to demonstrate compliance with safety and performance standards. Adequate testing is essential to prove device safety and effectiveness.
- Organize the Submission: Structure all collected information in a well-organized manner for submission. This involves arranging the documentation logically and coherently.
- Consider Pre-Submission Meeting: Though optional, a pre-submission meeting with the FDA can help clarify doubts and receive guidance on the application process.
- Submit Application: Use e-Submitter or FDA Unified Registration and Listing Systems (FURLS) to electronically submit the completed US FDA 510(k) application. The application should be complete and accurate.
- FDA Review Process: FDA will review the application for completeness and acceptance within about 2 weeks. Subsequent review times can vary, but the overall process takes several months.
- Address FDA Feedback: Be prepared to respond to any requests for additional information or clarifications during the FDA’s review. Thoroughly addressing FDA queries is essential.
- Obtain Clearance: Once the FDA is satisfied, they will issue a 510(k)-clearance letter, allowing you to market the device in the U.S. This clearance indicates that the device is deemed substantially equivalent to the predicate device.
Remember that the US FDA 510(k)-submission process may change over time, so it’s essential to refer to the latest FDA guidelines and regulations while preparing and submitting the application.
Need More Clarity on the US FDA 510(k) submission?
The US FDA 510(k) document:
A US FDA 510(k) document is a premarket submission made to the U.S. Food and Drug Administration (FDA) for certain medical devices, which requires device manufacturers to submit this type of application.
- Cover Letter: A brief introduction to the submission, providing a summary of the device and its intended use.
- Indications for Use: A clear statement of the intended use of the device, including the patient population and medical conditions it is intended to diagnose, treat, or monitor.
- Device Description: Detailed information about the design, materials, components, and specifications of the device.
- Substantial Equivalence Discussion: A comparison of the new device to the predicate device, highlighting similarities in design, technology, and intended use.
- Performance Data: Data and test results demonstrating that the device functions as intended and meets relevant safety and performance standards.
- Biocompatibility and Risk Analysis: Information on the device’s biocompatibility and an analysis of potential risks and hazards associated with its use.
- Labeling: The proposed labeling for the device, including instructions for use, warnings, and precautions.
- Sterilization and Packaging: Details on how the device will be sterilized and packaged for distribution.
- Clinical Data (if applicable): If the device raises new questions of safety or effectiveness, or if it’s not substantially equivalent to any predicate device, clinical data may be required to support its clearance.
The exact content and format of a US FDA 510(k) submission can vary depending on the device and its complexity. It’s important to consult the FDA’s official guidance documents and regulations when preparing a US FDA 510(k) submission to ensure that all necessary elements are included, and that the submission meets the FDA’s requirements.
Below image gives a high level understanding of the classification for medical devices as per US FDA.
What is a US FDA 510(k)-Predicate Device?
A predicate device refers to a device that is already on the market and your 510(k) submission should demonstrate that your device is very similar to it. Hence it is something which is –
- Legally marketed in the U.S. (510(k) clearance or PMA approval).
- Similar technology and intended use as the new device.
- Comparable design, materials, and construction.
- Demonstrated safety and effectiveness without significant concerns.
- Preferably recently cleared to align with current standards.
Choosing the Correct Regulatory Consultant for Collaboration:
The process of submitting a US FDA 510(k) can be difficult and complicated for companies, particularly those who are not familiar with the regulatory requirements. Partner with us, Operon Strategist, for a seamless US FDA 510(k) Submission. With our expertise and tailored approach, compliance becomes stress-free. Let us guide you through the process, so you can focus on advancing healthcare solutions. Reach out today and let’s make your medical device dreams a reality.
Need clarity on the 510(k)-submission process? Contact us Now.